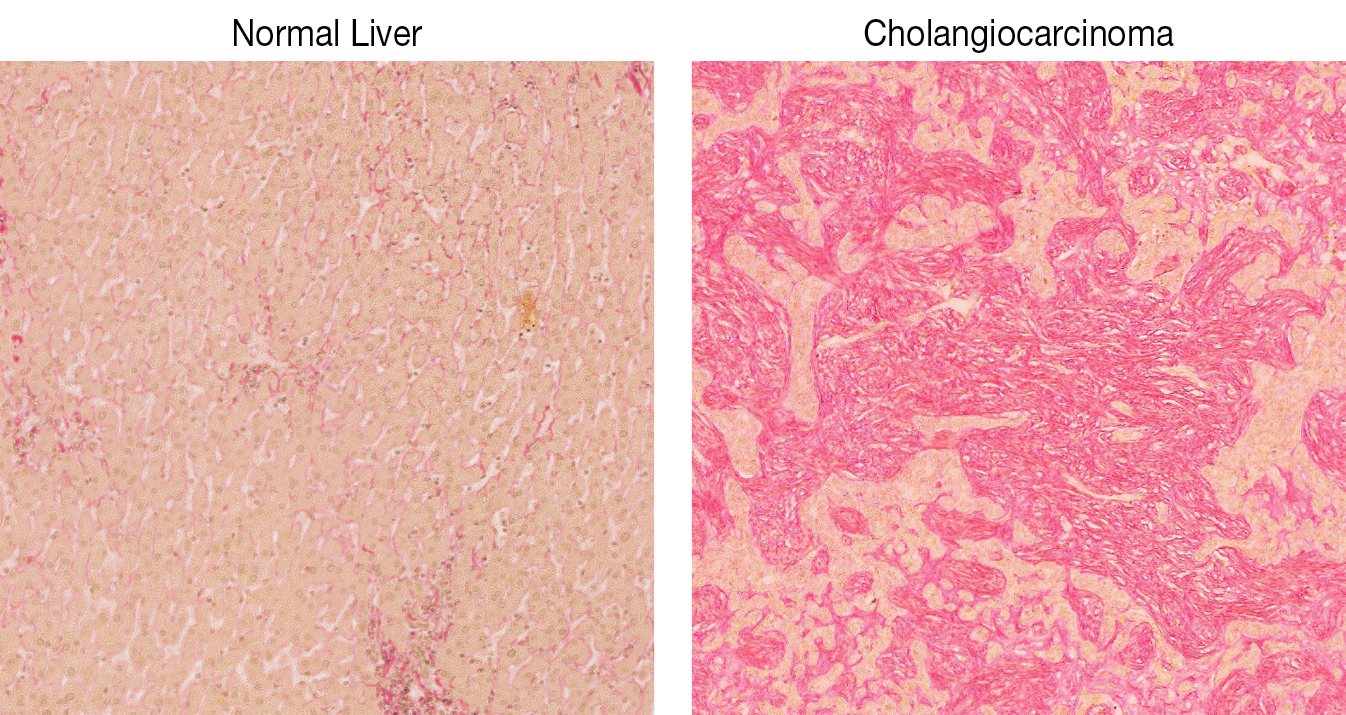
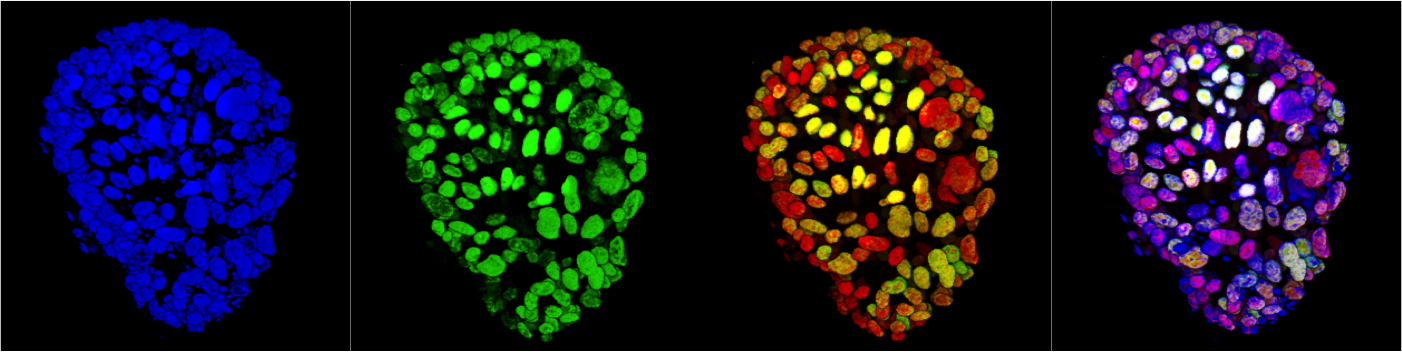
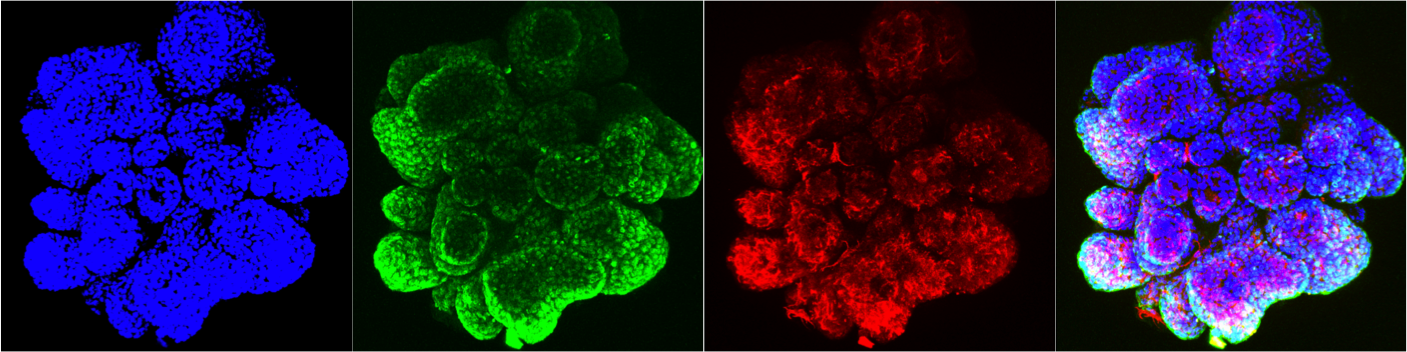
Tissue biology and tumorigenesis
Background
In living organs, cells are in intimate contact with each other and with their microenvironment. Within tissues, cells display remarkable, and yet mysterious, “social” behaviors. Prominent examples are the ability of cells to stop proliferating (i.e., of making copies of themselves) when they start touching each other; to change their destiny to accommodate tissue needs; or to self-organize into new tissue during the repair of damaged organs. Healthy tissues can also recognize individual tumor cells and either eliminate them or "normalize" their behavior, as such taming the effect of oncogenic mutations.
These tissue-level sensory and control systems are completely subverted in solid tumors, in favor of the expansion of renegade tumor cells. By understanding these overarching control systems, our ultimate goal is to devise strategies to eradicate established tumors and their metastases. For this, we are asking of what these tissue-level properties are made of. In the past 40 years, research on cancer has almost exclusively been driven by the view of cancer as a genetic disease. However, being a property exclusive of a system of cells, and not of the individual cell, tumor suppression at the tissue-level is not something directly encoded by our genes. Understanding tissue-level control of cell behavior in fact requires incorporation of new conceptual frameworks, by which information on the tissue’s own composition, physical structure, shape and architecture is perceived by each of its constituent cells.
In these years, we have contributed to such new frameworks with the discovery that YAP/TAZ, two highly related proteins regulating gene expression, are sensors of how cells, hard-wired within tissues, perceive themselves and their environment. Differently from all other known growth-regulating signals, YAP/TAZ activity is under control of cell's own shape and polarity. All these inputs are essentially physical and mechanical, influenced by the cell's attachment to other cells, from the landscape and rigidity of the extracellular matrix (ECM) in which cells are embedded. We recently discovered that YAP/TAZ activation endows cells with powers typical of stem-cells. In cancer, this translates YAP/TAZ activation into generation of new tumor-initiating cells, into tumor aggressiveness, chemoresistance, and metastasis. YAP/TAZ are indeed strikingly essential for tumorigenesis in most solid tumors and are pervasively activated in human malignancies, making YAP/TAZ biology an ideal target for cancer therapy.
IFOM was initially conceived as a network of scientists from the Italian main scientific centers. In line, our IFOM group operates within the Department of Molecular Medicine of the University of Padua School of Medicine, the N.1 biomedical research institution of the country according to all nation-wide evaluation exercises. Our perspectives and rationales are deeply embedded into IFOM own view on the need of a system-level understanding of cancer biology in order to develop ground-breaking curative approaches. Our approach, prior discoveries and research tools are not only in continuum with what pursued by other IFOM teams on "Mechano-oncology" themes, but is in fact synergic with those efforts. We work at the interface of different fields, with emphasis on translational medicine, including patients' datasets, modeling and treatment of human malignancies in advanced mouse models, human organoid biology and tumor organoids' characterization toward personalized medicine applications. This repertoire of approaches is complementary to the skills of the other IFOM teams. Conversely, IFOM expertise in advanced imaging, bioengineering and interdisciplinary exploration of the interface between cell biology, genome integrity and nuclear structure-function are critical for our own research endeavor, all in all allowing to pursue truly multi-disciplinary attack to some of the most devastating human malignancies.
We pursue three main research directions:
- To understand how the tissue context and in particular supracellular mechanical signals interact with oncogenic lesions to regulate cell behavior. Questions to be addressed include: are common oncogenic lesions allowing cells bearing them to disobey to the growth restraining mechanical signals of the surrounding normal tissues? Are tumors and metastases addicted to cellular mechanotransduction? Can we stop tumor growth in living tissues by modulating extracellular mechanical forces?
- To understand the intrinsic plasticity of normal and cancer cells at its epigenetic roots. Tumors have been originally described as "wounds that never heal", highlighting tumor emergence as a hijacking of ancient tissue repair mechanisms. What are the molecular determinants shared by tumor growth and organ re-growth during tissue damage? What are the differences? Can this knowledge be exploited to induce de novo regenerative potential in tissues wrecked havoc by disease, or, in the opposite direction, to pull-the-plug to the mechanisms that constantly rejuvenate the tumor? Are metastasis and chemoresistance a manifestation of the intrinsic "plasticity" of the cancer cells, imparting varying degrees of stemness, metabolic, and survival potentials?
- To study how combination of chemical and mechanical signals generates special niches fueling tumor progression, metastasis and chemoresistance. This includes interrogation of human tumor organoids from incurable cancers, and their validation as "avatars in the test-tube" of the human disease, and the use of engineered, defined substrates for 3D organoid cultures.
PhD project titles
PhD project titles
- Regulation of YAP/TAZ by cellular mechanotransduction
- Microenvironmental signals driving metastatic colonization
- Single cell characterization of human tumors through patient-derived organoids
- Cancer cell plasticity: epigenetic mechanisms, vulnerabilities and therapeutic opportunities
02 May 2018 rel.01
Photogallery
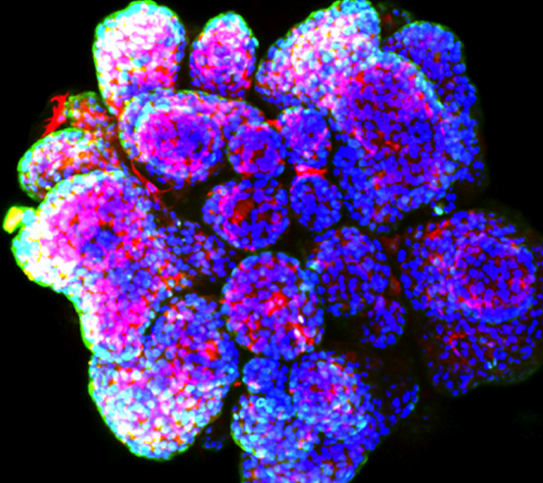 Mammary gland organoid
Mammary gland organoid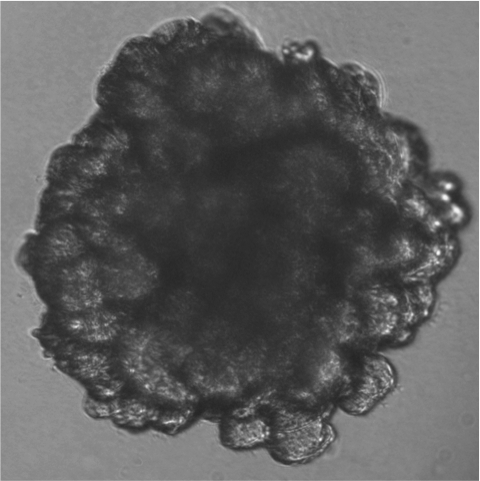 Human breast cancer organoid
Human breast cancer organoid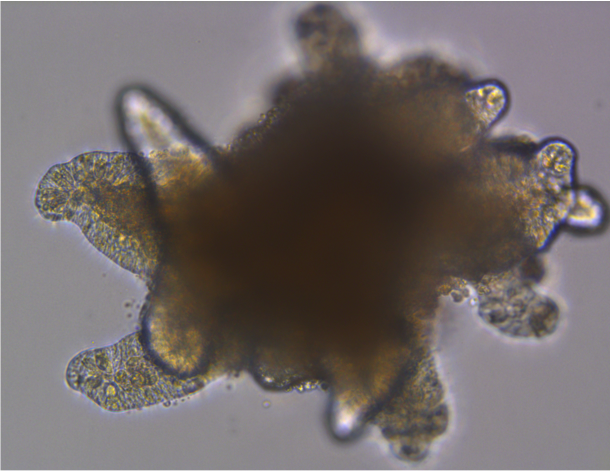 Intestinal Organoids
Intestinal Organoids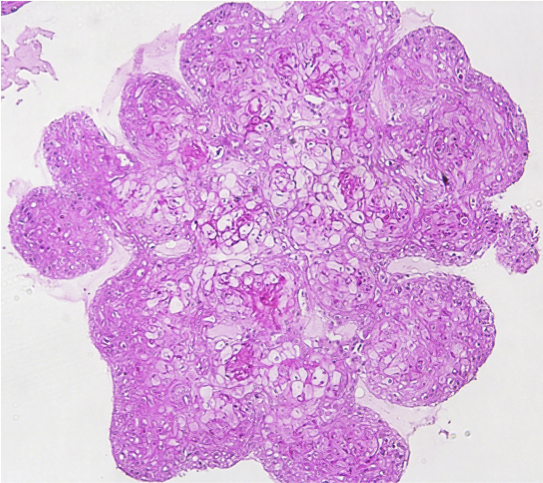 Human breast cancer organoid
Human breast cancer organoid