Cell Plasticity & Aging
Background
The concept of “cell plasticity” refers to the capacity of a cell to adopt different identities without any genetic mutations. A classic example of cell plasticity occurs during development, when embryonic stem cells differentiate into the immense diversity of cell types we see in the adult body. It is now appreciated that cell plasticity also plays an important role in adult tissues, particularly in the context of injury and regeneration, where it is indispensable for tissue repair. However, cell plasticity is also a salient feature of cancer and can contribute to tumorigenesis, resistance to therapy, and metastasis.
Our lab is interested in understanding and manipulating these dichotomous roles of cell plasticity. Our ultimate goal is to find ways to harness cell plasticity programs for enhanced tissue regeneration, and to control them in order to limit tumorigenesis, cancer progression, and relapse. To approach this, we use a combination of molecular and cell biology, and organoid models in vitro, in vivo mouse models, and next-generation sequencing technologies.
Ongoing Research Projects
1. The relationship between reparative cell plasticity and tumorigenesis
We are interested in understanding whether the cell plasticity that is activated during tissue repair is similar, different, or related to the cell plasticity that occurs in tumorigenesis. We are using colorectal cancer and intestinal injury models combined with lineage tracing systems to temporally identify, isolate, and study these plastic cells and their progeny. We are particularly interested in using single cell omics to understand the epigenetic profiles that define healthy and diseased states, and in combining this with metabolic interventions to manipulate plasticity. We also aim to discover novel approaches to disrupt cell plasticity in cancer for improved disease control.
2. Cell plasticity during aging
As we age, our regenerative capacity declines, while our cancer risk significantly increases. We are investigating whether changes in cell plasticity contribute to these age-associated phenomena. We are also using in vivo models of Yamanaka factor reprogramming, combined with lineage tracing tools, to study whether artificially induced cell plasticity affects aging regeneration, and cancer.
3. The role of senescent cells in aging and disease
Senescent cells are stressed or damaged cells that accumulate within our tissues as we age. While senescence is an important tumor suppressor and can promote tissue repair, inappropriate accumulation of senescent cells is pathogenic and contributes to the development or exacerbation of many age-associated diseases. We are studying the role of senescent cells in cell plasticity in the context of transformation, therapy resistance, and tissue regeneration. We are also developing novel senolytics - drugs that specifically target senescent cells - for the therapeutic elimination of senescent cells.
Photogallery
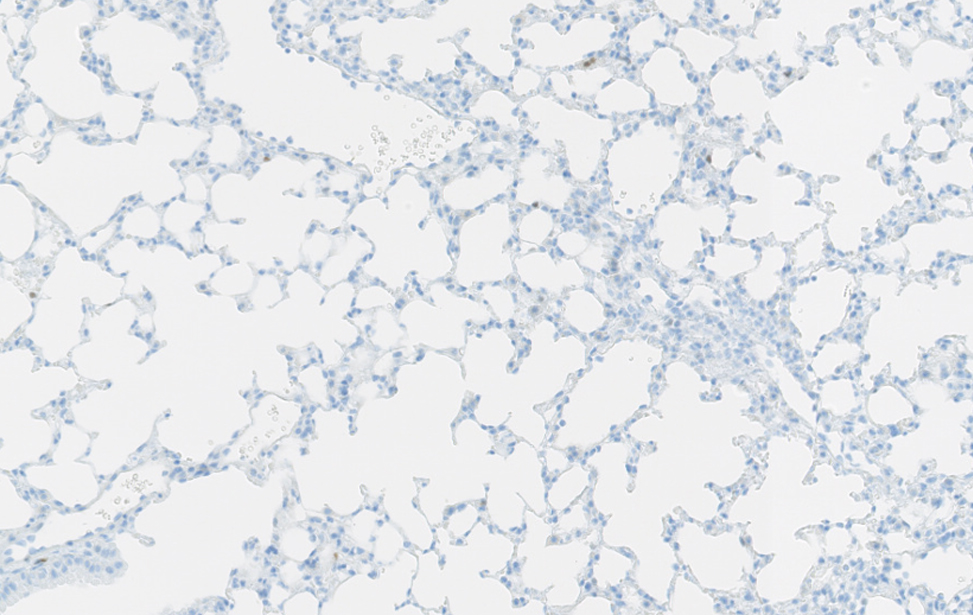 Polmone sano murino Immunoistochimica di un polmone sano di topo.
Polmone sano murino Immunoistochimica di un polmone sano di topo.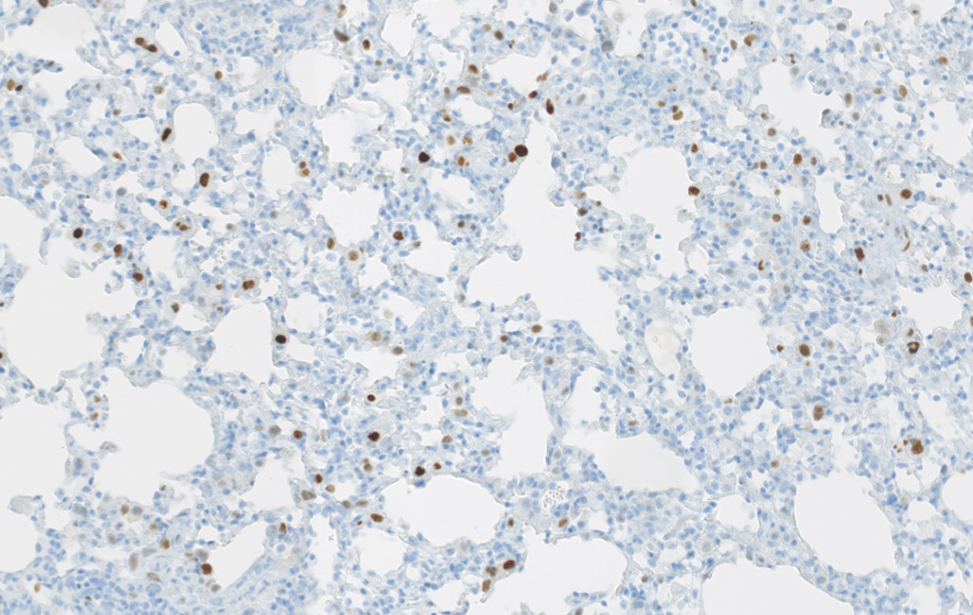 Polmone fibrotico Immunoistochimica che evidenzia le cellule senescenti (colore marrone) in un polmone fibrotico murino. Studiamo il contributo delle cellule senescenti agli stati fisiologici e patologici, e sviluppiamo trattamenti senolitici per eliminarle.
Polmone fibrotico Immunoistochimica che evidenzia le cellule senescenti (colore marrone) in un polmone fibrotico murino. Studiamo il contributo delle cellule senescenti agli stati fisiologici e patologici, e sviluppiamo trattamenti senolitici per eliminarle. 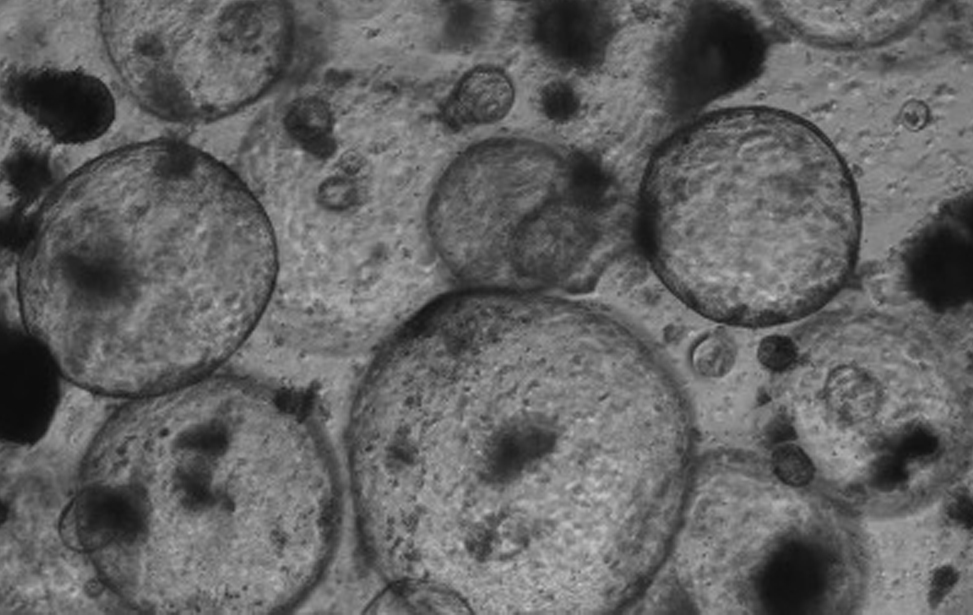 Organoidi derivati dal colon e dal pancreas murino, alcuni marcati con proteine fluorescenti rosse. Usiamo organoidi di topo e derivati da pazienti per studiare i cambiamenti molecolari che guidano la plasticità cellulare in situazioni di lesione, invecchiamento e cancro.
Organoidi derivati dal colon e dal pancreas murino, alcuni marcati con proteine fluorescenti rosse. Usiamo organoidi di topo e derivati da pazienti per studiare i cambiamenti molecolari che guidano la plasticità cellulare in situazioni di lesione, invecchiamento e cancro.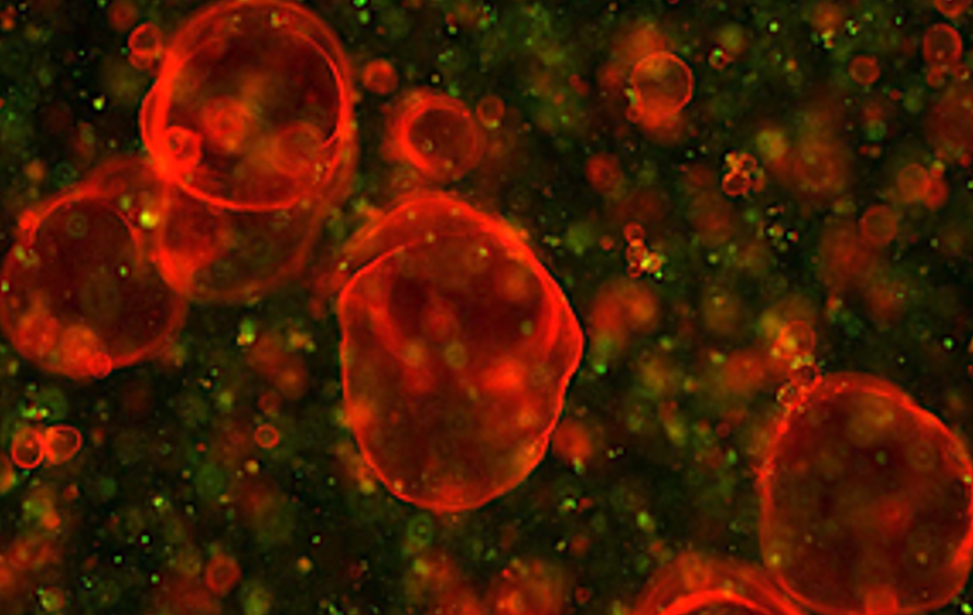 Organoidi derivati dal colon e dal pancreas murino, alcuni marcati con proteine fluorescenti rosse. Usiamo organoidi di topo e derivati da pazienti per studiare i cambiamenti molecolari che guidano la plasticità cellulare in situazioni di lesione, invecchiamento e cancro.
Organoidi derivati dal colon e dal pancreas murino, alcuni marcati con proteine fluorescenti rosse. Usiamo organoidi di topo e derivati da pazienti per studiare i cambiamenti molecolari che guidano la plasticità cellulare in situazioni di lesione, invecchiamento e cancro.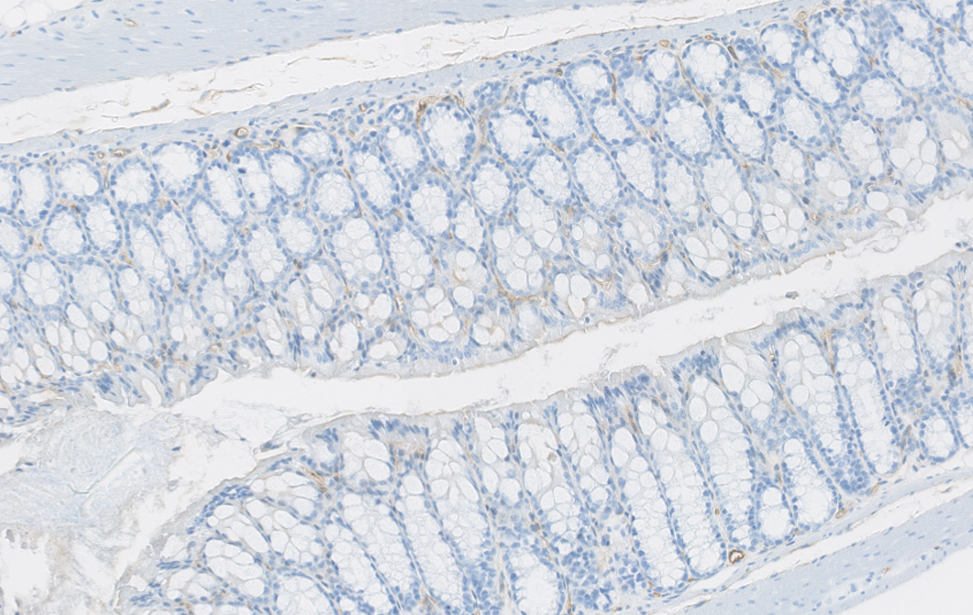 Colon sano vs. colite ulcerosa – Immunoistochimica che mostra la plasticità cellulare indotta da lesione (colore marrone) nel colon murino. Studiamo come la plasticità cellulare contribuisce alla riparazione, all’invecchiamento e allo sviluppo del cancro.
Colon sano vs. colite ulcerosa – Immunoistochimica che mostra la plasticità cellulare indotta da lesione (colore marrone) nel colon murino. Studiamo come la plasticità cellulare contribuisce alla riparazione, all’invecchiamento e allo sviluppo del cancro.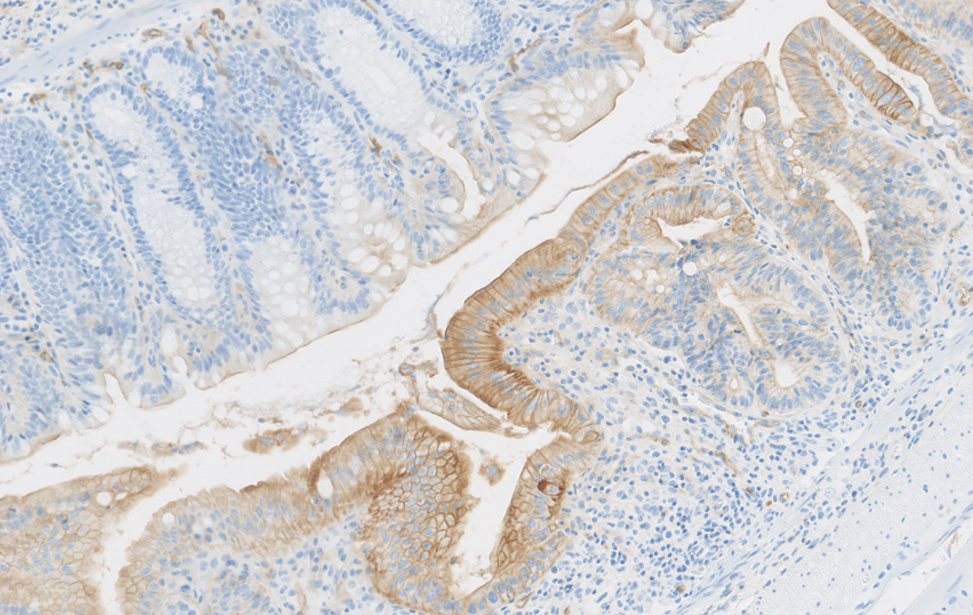
Colite Ulcerosa
Immunoistochimica della plasticità cellulare indotta da danno (colore marrone) nel colon murino durante la colite ulcerosa.
Studiamo il ruolo della plasticità cellulare nella riparazione dei tessuti, nell’invecchiamento e nello sviluppo del cancro.
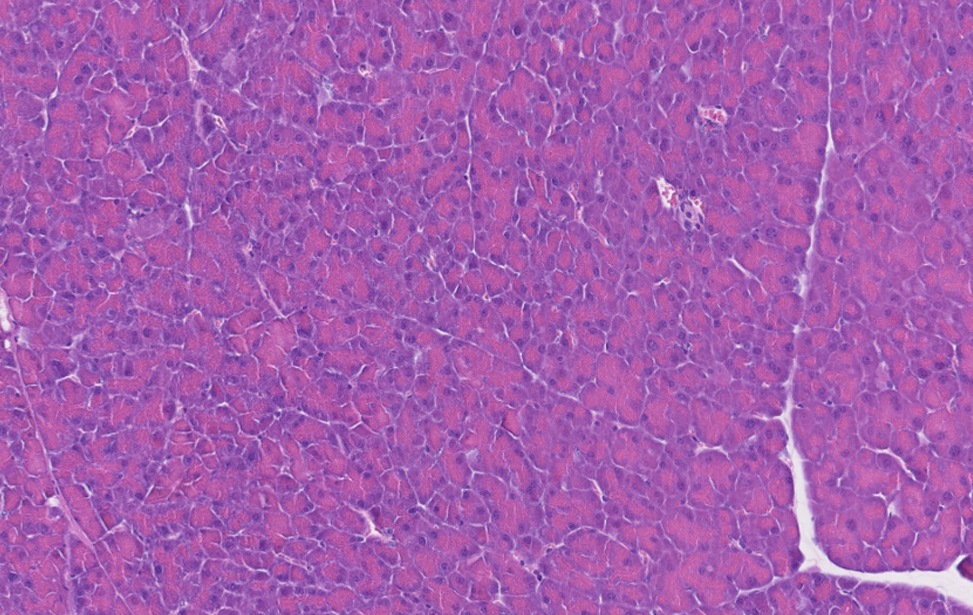
Pancreas Normale
Immunoistochimica di un pancreas murino sano.
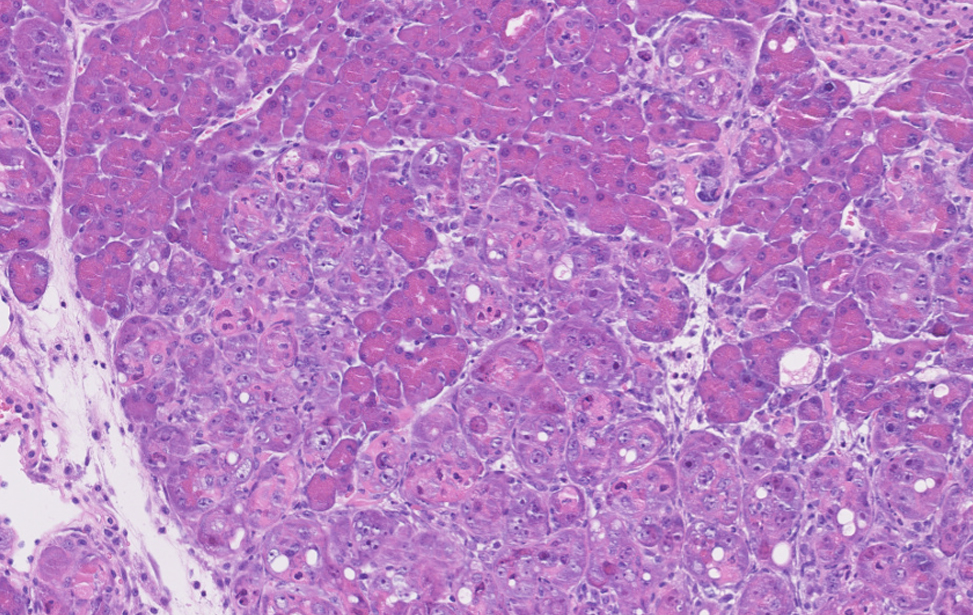
Pancreas Riprogrammato
Immunoistochimica di un pancreas murino riprogrammato mediante l'espressione dei fattori di Yamanaka (OSKM).
La plasticità cellulare indotta da OSKM condivide somiglianze con quella indotta da danno, e la utilizziamo come strumento per comprendere come possa essere controllata.
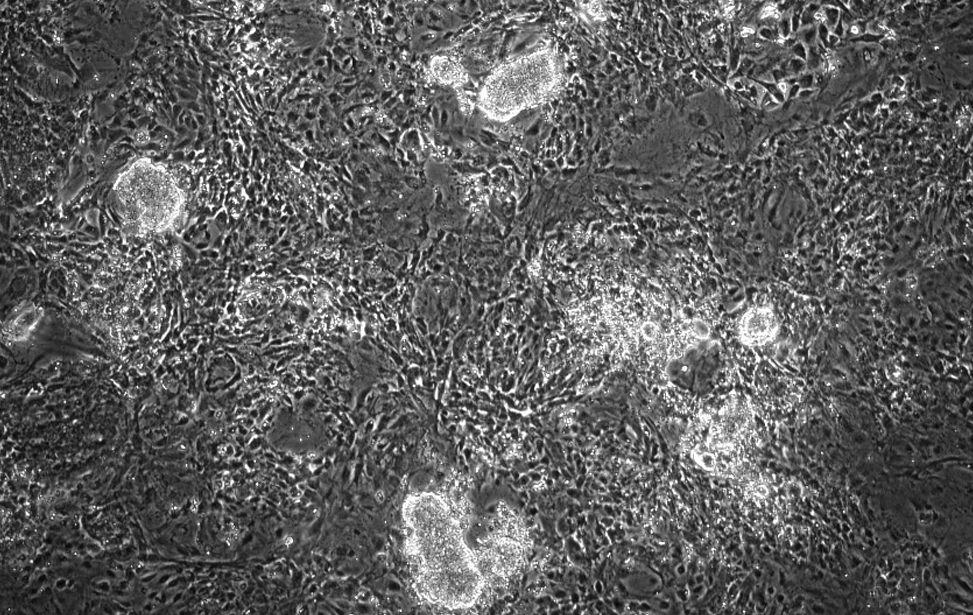
iPSC
Cellule staminali pluripotenti indotte (colonie brillanti e rotonde) generate da fibroblasti murini mediante espressione di OSKM.
Usiamo la plasticità cellulare indotta da OSKM per comprendere i meccanismi che regolano la plasticità cellulare, in parallelo a quella indotta da danno.
